Abstract

Introduction: Dasatinib has demonstrated superior responses versus imatinib in patients (pts) with newly diagnosed chronic myeloid leukemia in the chronic phase (CML-CP) (Cortes JCO 2016). Achievement of early molecular response (EMR; BCR::ABL1 ≤ 10% International Scale [IS]) at 3 months increases the probability of subsequent deep molecular response (DMR) and improves survival (Cortes JCO 2016, Hochhaus Leukemia 2016). However, ⅓ of pts treated with imatinib did not reach an EMR (Hughes Blood 2014); there are no standard treatment strategies for these pts. DASCERN (NCT01593254) is the first prospective, randomized, phase 2b trial to demonstrate the potential benefit of switching early to dasatinib in pts with no EMR after 3 months of initiating imatinib. At 12 months, pts randomized to dasatinib had significantly higher major molecular response (MMR) rates versus imatinib (29% vs 13%, primary endpoint) (Cortes Leukemia 2020). At 3 years, cumulative MMR rates were 67% and 40% with dasatinib and imatinib, respectively (Cortes EHA 2020 EP756). Switching to dasatinib did not increase the incidence of treatment-emergent adverse events (AEs). Here we report the results from the final 5-year analysis.
Methods: Pts with complete hematologic response but no EMR at 3 months after initiating imatinib 400 mg were randomized 2:1 to an early switch to dasatinib (100 mg) or continue imatinib (≥ 400 mg). Pts who experienced subsequent failure with imatinib (per European LeukemiaNet 2013 recommendations at the time of the study) crossed over to dasatinib. The intent-to-treat (ITT) population included all pts randomized to each arm, irrespective of crossover status. Study endpoints included time to MMR and molecular response 4.5 (MR4.5; 4.5-log reduction in BCR::ABL1 levels; ≤ 0.0032% IS), progression-free survival (PFS), overall survival (OS), and safety.
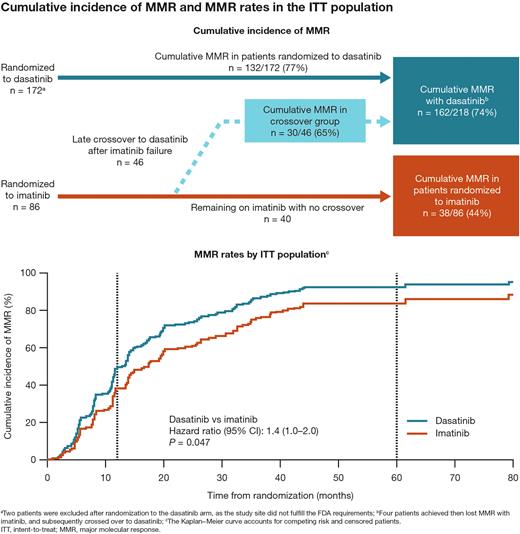
Results: At a median follow-up of 80 months (data cutoff, April 24, 2022), 174 and 86 pts with no EMR after imatinib were randomized to dasatinib and imatinib, respectively; 46 (53%) imatinib-randomized pts experienced subsequent imatinib failure and crossed over to dasatinib in a median of 9.5 months. Detailed baseline characteristics have been previously reported (Cortes EHA 2020 EP756). The median age was 37 years, and 73% of patients were Asian. All pts discontinued treatment, mainly due to study drug toxicity (26 [10%]), and other reasons (176 [68%], mostly study completion); 6 (2%) discontinued due to death. The MMR rate by 5 years in the ITT population was 77% and 44%, respectively, for pts in the dasatinib and imatinib arms. After accounting for crossover, cumulative MMR rates were 74% with dasatinib and 44% with imatinib; 65% of pts who crossed over from imatinib to dasatinib achieved MMR (Figure). Across the 5-year study period, more pts randomized to dasatinib than imatinib achieved MR4 and MR4.5 (MR4, 52% vs 31%; MR4.5, 36% vs 26%). Only 1/46 pts who later crossed over to dasatinib after imatinib failure achieved MR4.5. PFS in the ITT population was 94% in both treatment arms. PFS was numerically lower in pts who later crossed over to dasatinib after imatinib failure (90%). OS in the ITT population was similar between both arms (96% vs 95%). OS in pts who crossed over to dasatinib was 94%. Six additional deaths were reported since the 3-year follow-up. Any grade treatment-related AEs were experienced by 149 pts (87%) randomized to dasatinib, 69 (80%) randomized to imatinib, and 37 patients (80%) who crossed over to dasatinib. Treatment-related pleural effusion occurred in 31 pts (18%) randomized to dasatinib (n = 6 grade 3/4), 9 pts (20%) who crossed over to dasatinib after imatinib failure (n = 2 grade 3/4), and 0 pts who remained on imatinib (no crossover).
Conclusions: At the final 5-year follow-up, sustained clinical benefit was observed from an early switch to dasatinib after suboptimal responses to 3 months of imatinib. MMR and DMR rates were higher in pts randomized to dasatinib versus imatinib. Overall, the safety profile was consistent with previous reports. These results complement the previous findings that switching to dasatinib promptly after a lack of EMR with imatinib increases the chance of achieving DMR, which may improve outcomes in pts with CML-CP. Therefore, this study further highlights the significance of achieving optimal responses early during treatment.
Study support: Funded by Bristol Myers Squibb.
Disclosures
Cortes:Kartos: Research Funding; Forma Therapuetic: Consultancy; Pfizer: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Sun Pharma: Consultancy, Research Funding; Gilead: Consultancy; Abbvie: Consultancy, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Biopath Holdings: Consultancy, Current equity holder in private company; Bristol Myers Squibb: Consultancy, Research Funding. Wang:Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy. Hochhaus:Bristol Myers Squibb: Research Funding; Novartis: Research Funding; Pfizer: Research Funding; Incyte: Research Funding. Kim:Bristol Myers Squibb: Consultancy, Honoraria, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; Pfizer: Consultancy, Honoraria, Research Funding, Speakers Bureau; Otsuka: Consultancy, Honoraria, Research Funding, Speakers Bureau; Il-Yang: Consultancy, Honoraria, Research Funding, Speakers Bureau. Radich:Novartis: Other; Amgen: Other; Nuprobe: Other; Cepheid: Other; HTG: Other. Savona:Astex Pharmaceuticals: Research Funding; Taiho Pharmaceutical: Consultancy; Sierra Oncology: Consultancy, Other: travel expenses; TG Therapeutics: Consultancy, Other: Travel expenses, Research Funding; Karyopharm Therapeutics: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Geron: Consultancy; Takeda: Consultancy; Incyte Corporation: Research Funding; Forma: Consultancy; Novartis: Consultancy; Ryvu Therapeutics: Consultancy, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Other: travel expenses; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: travel expenses; ALX Oncology: Research Funding. Martin-Regueira:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Sy:Bristol Myers Squibb: Current Employment. Saglio:Novartis: Speakers Bureau.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal